Partial structure factors
There are a few, somewhat different definitions of partials
Faber-Ziman definition/formalism
One way used to define the partial structure factors has been proposed by Faber and Ziman [1]. In this approach the structure factor is represented by the correlations between the different chemical species. To describe the correlation between the
The total structure factor is then obtained by the relation:
Ashcroft-Langreth definition/formalism
In a similar approach, based on the correlation between the chemical species, and developed by Ashcroft and Langreth [2], [3], [4], the partial structure factors
Then the total structure factor can be calculated using:
Bhatia-Thornton definition/formalism
In this approach, used in the case of binary systems AB
-
Its Fourier transform allows to obtain a global description of the structure of the solid, ie. of the distribution of the experimental scattering centers, or atomic nuclei, positions. The nature of the chemical species spread in the scattering centers is not considered. Furthermore if -
Its Fourier transform allows to obtain an idea of the distribution of the chemical species over the scattering centers described using the
-
Its Fourier transform allows to obtain a correlation between the scattering centers and their occupation by a given chemical species. The more the chemical species related partial structure factors are different (
If we consider the binary mixture as an ionic mixture then it is possible to calculate the Charge-Charge
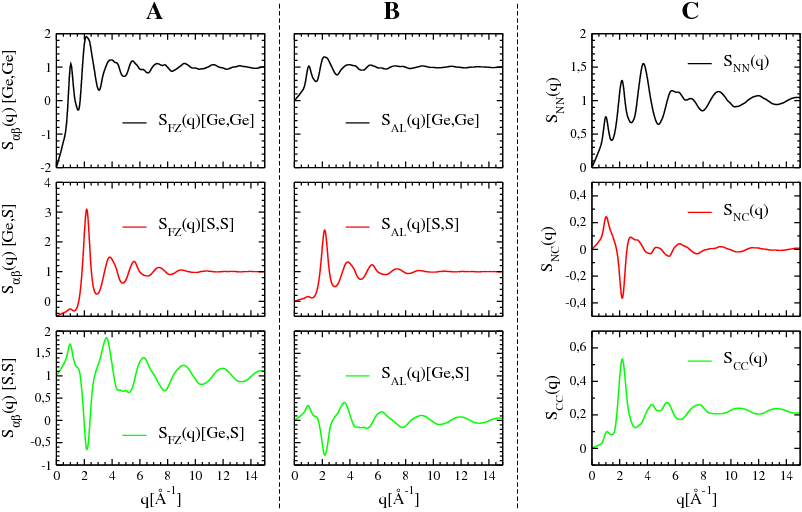
Figure 5.6 illustrates, and allows to compare, the partial structure factors of glassy GeS
Particles that can be described using spheres of the same diameter and occupying the same molar volume, subject to the same thermal constrains, in a mixture where the substitution energy of a particle by another is equal to zero.↩︎
- T. E. Faber and Z. J. M., Phil. Mag., vol. 11, no. 109, pp. 153–173, 1965.
- N. W. Ashcroft and D. C. Langreth, Phys. Rev., vol. 156, no. 3, pp. 685–692, 1967.
- N. W. Ashcroft and D. C. Langreth, Phys. Rev., vol. 159, no. 3, pp. 500–510, 1967.
- N. W. Ashcroft and D. C. Langreth, Phys. Rev., vol. 166, no. 3, p. 934, 1968.
- A. B. Bhatia and D. E. Thornton, Phys. Rev. B., vol. 2, no. 8, pp. 3004–3012, 1970.